Health, Psychedelics, Science
Psychedelics and Neuroplasticity: Exploring the Biological Mechanisms
Microdosing
In recent years, microdosing psilocybin has become a popular trend among those looking to improve their mental health and well-being. The practice involves taking small doses of psilocybin, the active ingredient in “magic mushrooms,” on a regular basis to enjoy the benefits without the full “psychedelic” experience.
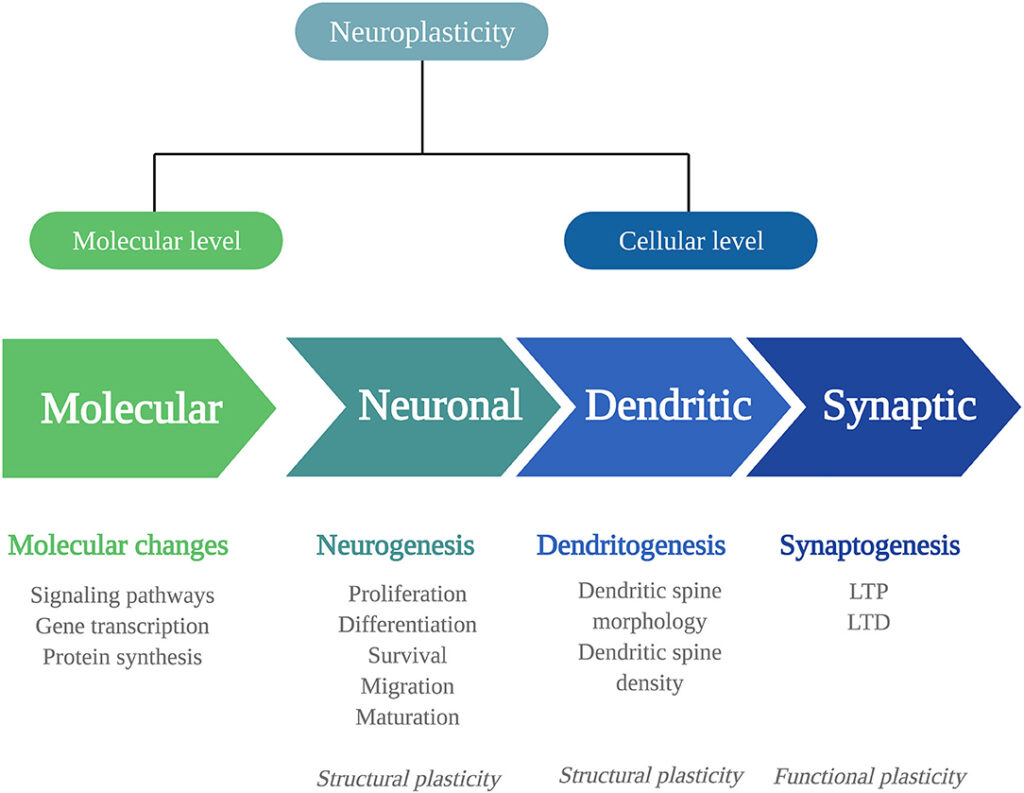
One of the reasons why microdosing psilocybin is thought to be beneficial for mental health is because of its effects on the brain’s neurogenesis and neuroplasticity. Neurogenesis refers to the process of creating new brain cells, while neuroplasticity refers to the brain’s ability to change and reorganize itself as a reaction to new experiences and learning.
Therapeutic Potential of psychedelics
Recent studies have demonstrated that psychedelics such as ayahuasca, DMT, psilocybin, and LSD offer therapeutic benefits for stress-related conditions. These substances can enhance cognitive abilities, alleviate depression, reduce anxiety, and address addiction. These improvements may result from alterations in brain neuroplasticity.
This review’s primary objective is to summarize the current evidence regarding how psychedelics impact the brain at a cellular and molecular level. It will explore these effects following both single and repeated usage of psychedelics. Furthermore, it identifies areas where we need a deeper understanding of how the biology of psychedelics relates to clinical outcomes and potential therapeutic advantages.
Single Administration of Psychedelics:
- Studies have shown that a single administration of psychedelics produces rapid changes in plasticity mechanisms at the molecular, neuronal, synaptic, and dendritic levels.
- The expression of plasticity-related genes and proteins, such as Brain-Derived Neurotrophic Factor (BDNF), is altered after a single administration, leading to changes in neuroplasticity.
- Dendritic complexity is increased, and these effects can outlast the acute psychedelic experience.
Repeated Administration of Psychedelics:
- Repeated administration of psychedelics directly stimulates neurogenesis (the birth of new neurons) and increases BDNF mRNA levels, even up to a month after treatment.
- These findings suggest that psychedelics induce long-lasting molecular and cellular adaptations related to neuroplasticity.
Behavioral Effects:
- Limited studies have explored the relationship between biological and behavioral adaptations induced by psychedelics.
- Some studies have shown increased learning behavior in response to psychedelic treatment.
- Ayahuasca-induced increases in plasma BDNF levels were correlated with reduced depressive symptoms in clinical populations.
Knowledge Gaps:
- The specific cellular mechanisms activated by different psychedelics and their relationship to long-term clinical and biological effects require further investigation.
- The role of non-psychedelic compounds present in ayahuasca, such as harmine, tetrahydroharmine, and harmaline, in neuroplasticity changes should be considered.
Molecular and cellular adaptations
This highlights the evidence: psychedelics induce molecular and cellular adaptations connected to neuroplasticity. Consequently, the observed changes could underlie the therapeutic effects of psychedelics in stress-related disorders.
Moreover, future research needs to prioritize decoding the specific biological mechanisms activated by different psychedelics. It should also explore their relationship to long-term clinical and biological effects.
In essence, this knowledge will contribute significantly to understanding the therapeutic potential of psychedelics. It will facilitate their integration into evidence-based treatments for mental health disorders.
Source: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.724606/full